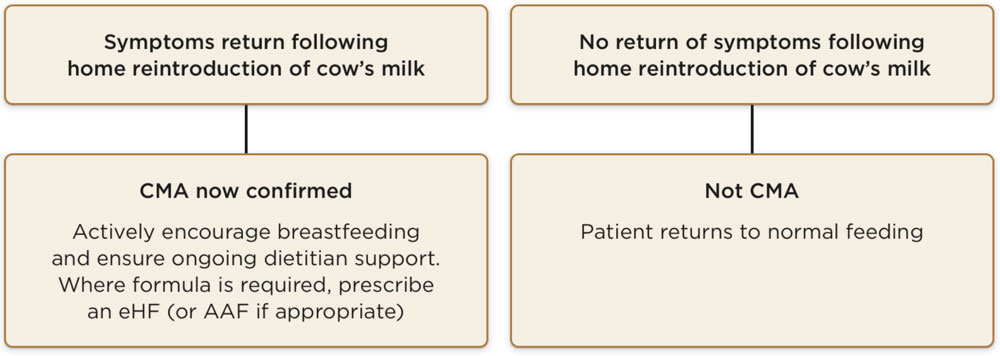
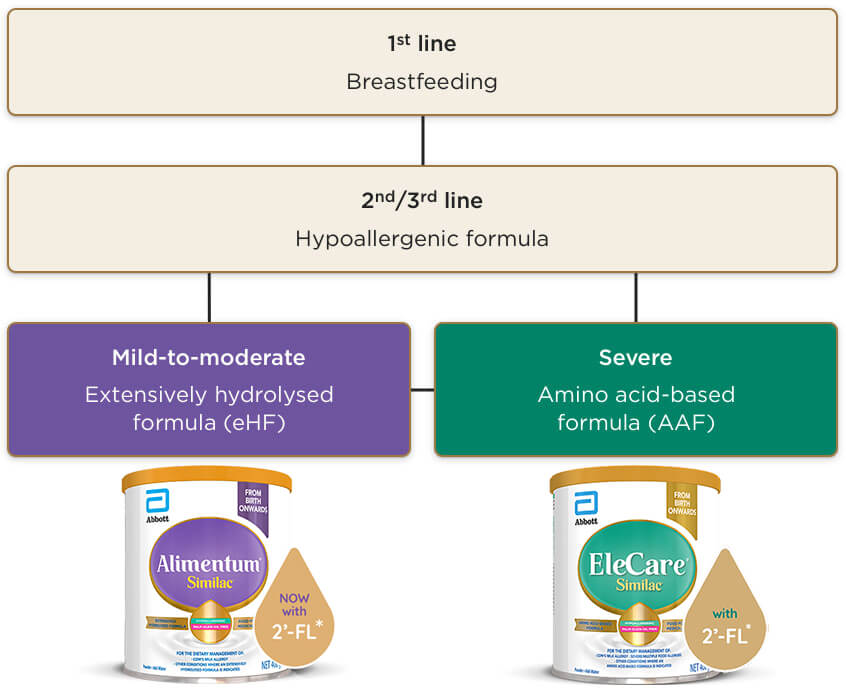
Exclusive breastfeeding is the first choice recommendation until 6 months, and this should be actively encouraged and supported:*1
- •If an exclusively breastfed infant is symptomatic, then a strict elimination of cow’s milk containing foods from the maternal diet with daily supplements of calcium and vitamin D is recommended (early support from a dietitian is advised to facilitate this)1–3
If exclusive breastfeeding is not possible or the mother chooses a mixed feeding approach, then a trial of hypoallergenic formula should be initiated:1–3
- •In most cases, an extensively hydrolysed formula (eHF) should be recommended, while amino acid-based formulas (AAF) are used if an eHF is not well tolerated and for the management of severe cases1–3
- •If mixed feeding, the mother can continue to consume cow’s milk protein, but if her child’s symptoms persist, she should exclude it from her diet (with the support of a dietitian)1,3
Breast milk provides the nutrition and immune support infants need to face their first adventures.4–6